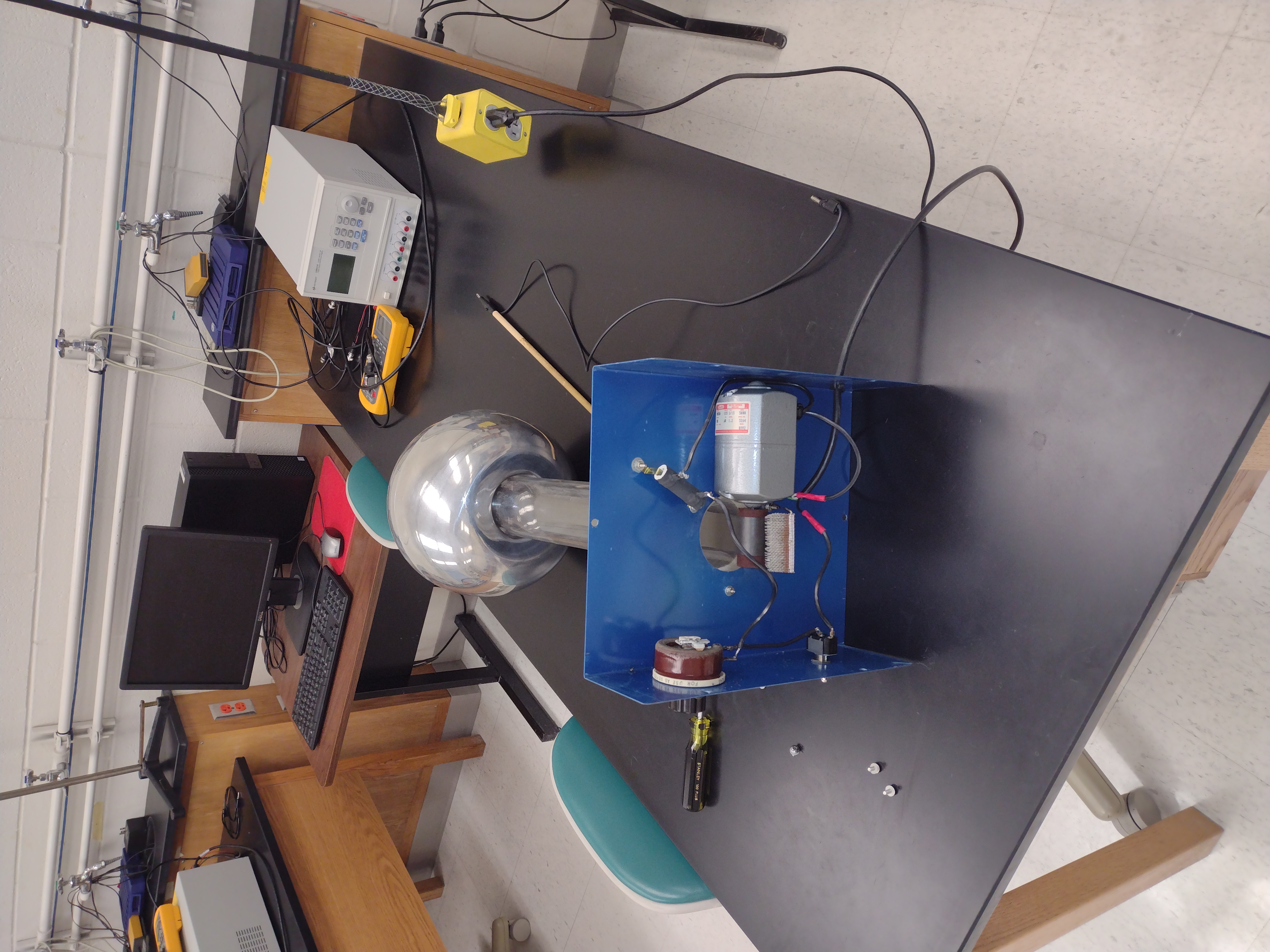
Demo: Van de Graaff Generator



Electric Charge
You should have already taken the baseline quiz for Module 1.
Did anybody have trouble?
Charge





How much charge is a lot? What if we had 1 coulomb of charge on the ping pong ball and 2 coulomb of charge on the Van de Graff generator. What would the force be if they were 1 meter apart?
So how much charge is on the ping pong ball?
\[ Q_1 = \frac{R_1^2 R_2^2}{R_1 + R_2} V \sum_{n=1}^\infty \frac{1}{n(n - R_1/(R_1 + R_2))} \frac{Q_T}{C} \] Lekner 2012
Most text books use the fact that the electric potential is the same for both spheres to derive \[ \frac{Q_1}{R_1} = \frac{Q_2}{R_2} \rightarrow Q_1^\prime = \frac{R_1}{R_1 + R_2} Q_T \]
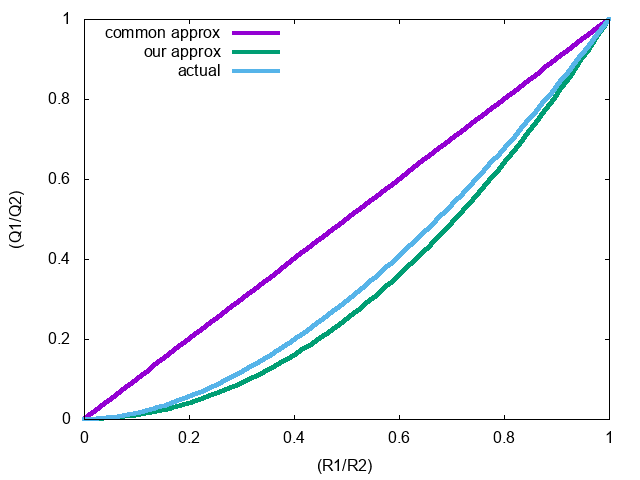
This is also an approximation…
Actual \[ \frac{Q_1}{Q_2} = \left(\frac{R_1}{R_2}\right)^2 \left(\frac{\pi^2}{6} \right)^{(R_2-R_1)/(R_1+R_2)} \]
Our Approximation \[ \frac{Q_1}{Q_2} = \frac{R_1^2}{R_2^2} = \left( \frac{R_1}{R_2} \right)^2 \]
Common Approximation \[ \frac{Q_1}{Q_2} = \frac{R_1}{R_2} \]
If we have some total charge \(Q_T\) that is split between two objects, the force between the two objects depends on how the charge is split. What distribution gives the largest force?
What if we had 3 spheres? What would the force on one of the spheres be?
1663 - electrostatic generator by Otto von Guericke
1785 - Coulomb’s Law confirmed
1803 - Atomic theory - John Dalton
1897 - Electron discovered - J.J. Thomson
1904 - Plum pudding model of atom - J.J. Thomson
1911 - Atomic nucleus discovered - Ernest Rutherford
1913 - Bohr model of the atom
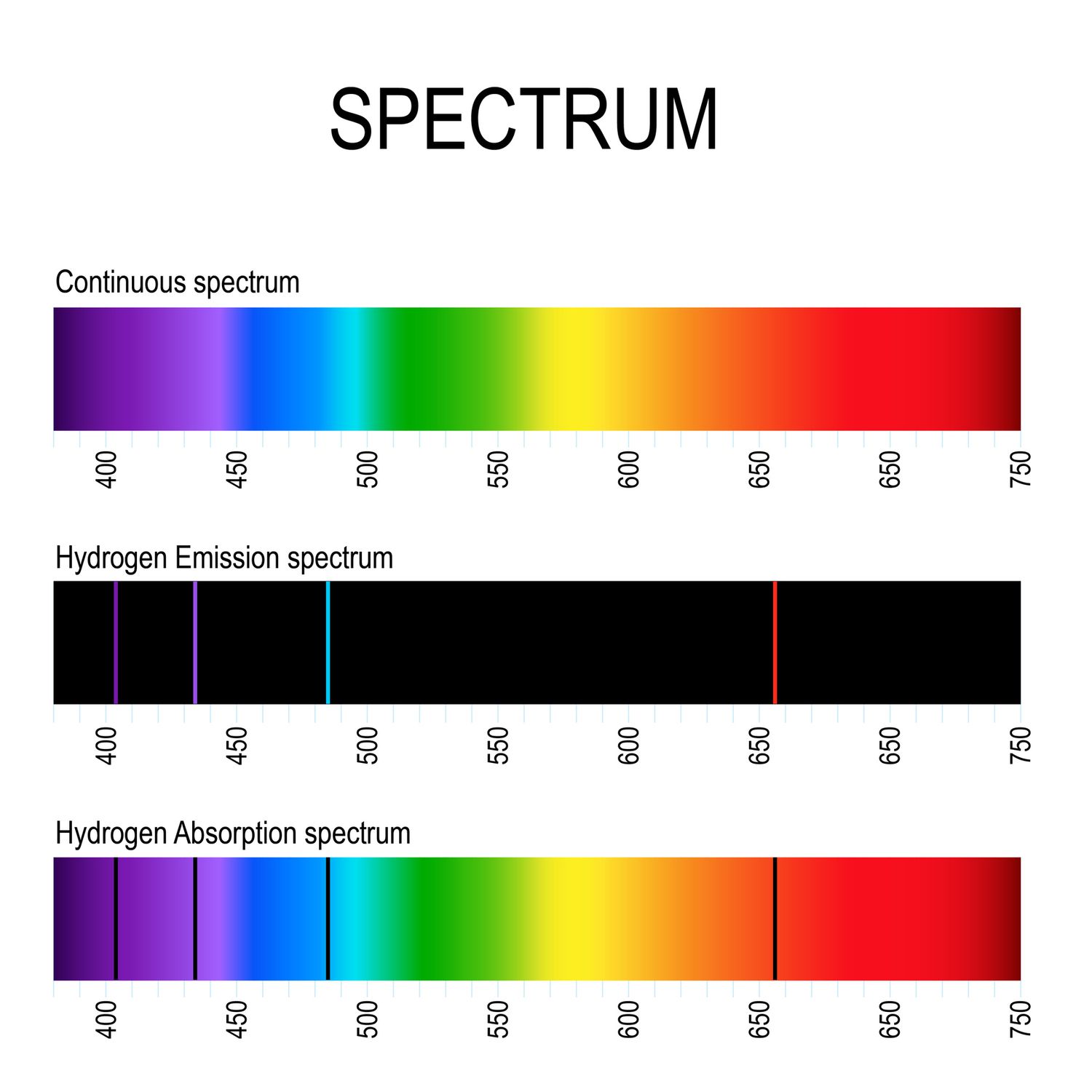
If an atom is a positively charged nucleus surrounded by negatively charged electrons, perhaps the electrons just orbit around the nucleus like a planet around the Sun…
How fast would the electron be traveling in such an orbit?
What would the acceleration be?
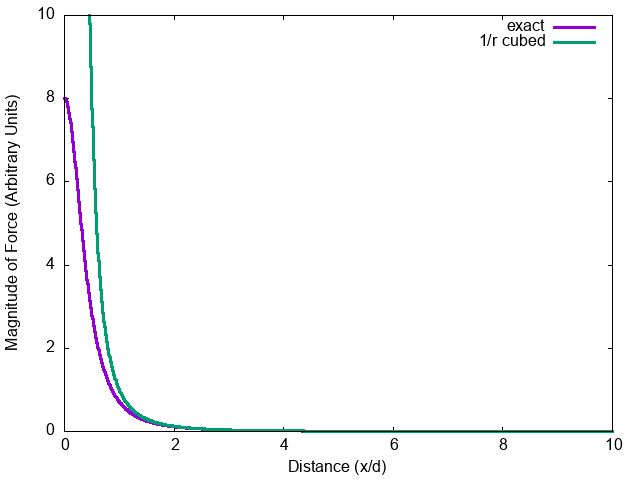

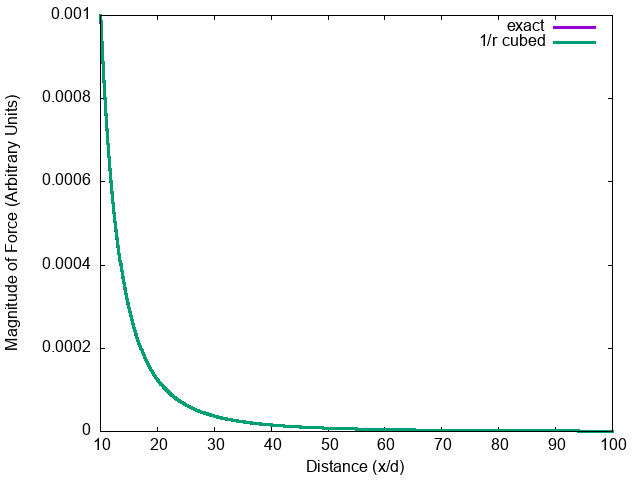
Force on a dipole by a dipole.
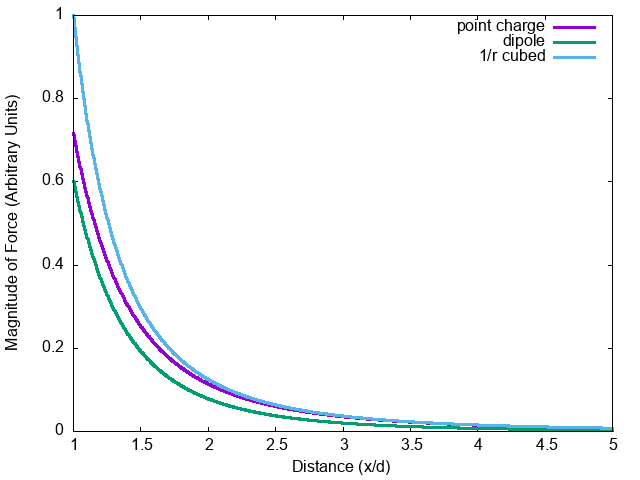
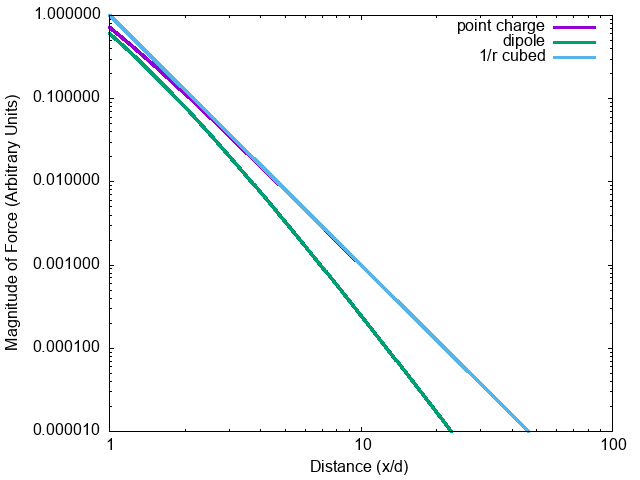
What is the net force on \(q_1\)?
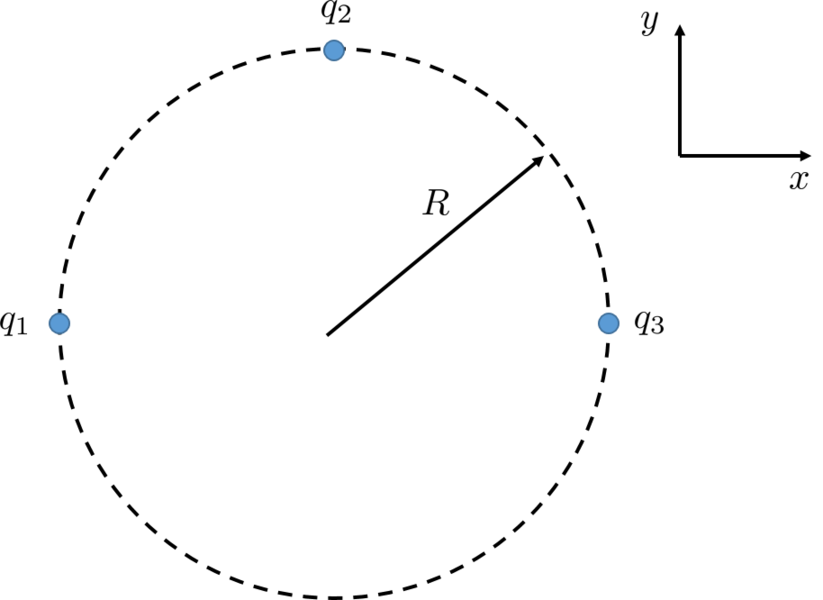
What is the net force on \(q_1\)?
